Early clinical effects of Huntington's disease new drug, Roche took over development
Early clinical effects of Huntington's disease new drug, Roche took over development
December 14, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Ionis Pharmaceuticals announced that the company's new drug IONIS-HTTRx for the treatment of Huntington's Disease (HD) significantly reduced the production of mutant huntingtin protein (mHTT) in patients in the clinical 1/2a phase. Therefore, Ionis partner Roche will exercise its power to develop IONIS-HTTRx from Ionis and conduct further clinical development.

HD is a rare genetic neurodegenerative disease in which the cognitive and motor skills of HD patients gradually decline and eventually die of pneumonia, heart failure or other complications. There are approximately 30,000 HD patients in the United States, and 200,000 people are at risk of developing HD. The cause of HD is due to the trinucleotide sequence amplification of the gene encoding HTT, which results in the production of mHTT protein which is toxic and progressively damages neurons in the brain. At present, existing therapies can only alleviate the symptoms of HD, and no treatment to change the progression of HD disease has been approved by the FDA.
The IONIS-HTTRx, jointly developed by Ionis and Roche, is an antisense innovative therapy that targets mRNA encoding the HTT protein. By binding to mRNA encoding the HTT protein, IONIS-HTTRx is able to reduce the occurrence of all HTT protein translations, so the drug can reduce the production of toxic mHTT regardless of where the gene mutation encoding HTT occurs. It has been granted orphan drug status by the US FDA and the European Medicines Agency.
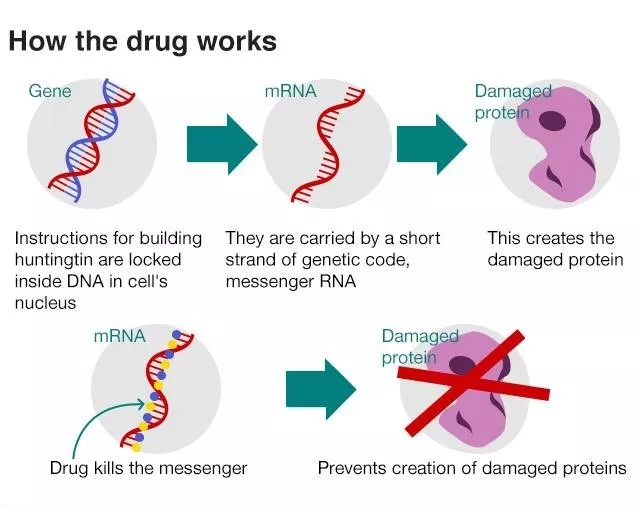
â–² Working Mechanism of New Drugs against Huntington's Disease (Source: University College London)
In this placebo-controlled, randomized dose escalation clinical 1/2a trial, 46 HD patients were treated with HTTRx. The level of mHTT in HD patients treated with HTTRx decreased correspondingly with increasing doses of the drug received. At the same time, the safety and tolerability characteristics of IONIS-HTTRx in this clinical 1/2a trial support Ionis and Roche to further develop it.
Under the agreement, Roche will pay $45 million to take over all subsequent developments of IONIS-HTTRx. Ionis and Roche will publish the full results of this clinical trial at the medical conference in the first half of 2018 and plan to publish the results in medical journals. For patients who completed the clinical 1/2a trial, Ionis and Roche recently launched an open-label extension trial to continue to evaluate the long-term efficacy of IONIS-HTTRx in them.

â–² Dr. Sarah Tabrizi (Source: University College London)
"The results of this clinical trial are groundbreaking for HD patients and their families. For the first time, there is a drug that reduces the level of protein that causes disease in the nervous system, and it shows good safety and resistance. Responsiveness. The key now is to rapidly conduct larger-scale clinical trials to determine whether IONIS-HTTRx can delay the progression of HD disease," said the head of the clinical 1/2a trial, University College London HD Center (University College London) Dr. Sarah Tabrizi, Director of Clinical Neurology at Huntington Center, said.
Reference materials:
[1] Ionis Pharmaceuticals Licenses IONIS-HTT Rx to Partner Following Successful Phase 1/2a Study in Patients with Huntington's Disease
[2] Huntington's breakthrough may stop disease
Anchor Rods & Grounding Rods
Thimble Eye, Twin Eye, Triple Eye and Oval Eye Anchor Rods are used for guying with expanding and cross plate anchros. Hot dip galvanized to meet ASTM A153 specification.
No Wrench Anchor are used for guying wires at utility poles. Eye dimensions are the same as Anchor Rods.
Expanding Anchor are made for installation in hole augered by power drillers. A retainer on the bottom holds the nut from the forged eye rod. Black Painting or Hot dip galvanized finish.
Ground Rods are used as a grounding electrode for building and poles. These rods have a cone point for easy driving and a plain end for attaching a ground rod clamp. Hot Dip Galvanized or Copper plating finish.
Extension Anchor Rod used in conjunction with internally tapped anchors and couplings or eye nuts.

Anchoring Grounding,Galvanized Ground Rod,Copper Bonded Earth Rod,Rock Anchor Extension Rod
Ningbo Yokelink Machinery Co.,Limited , https://www.yokelink.com