Edge's new formulation of nimodipine EG-1962 clinical phase III failure will be significantly reduced
Edge's new formulation of nimodipine EG-1962 clinical phase III failure will be significantly reduced
March 30, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Edge Therapeutics' lead drug EG-1962 has recently revived a reversal, failing to reproduce the positive results of early stage I/II clinical trials in a phase III clinical trial of aortic subarachnoid hemorrhage (aSAH) The stock price plummeted.

Just recently, Edge announced the interim analysis of the EG-1962 Phase III clinical study NEWTON-2. The study was conducted in aSAH adult patients and evaluated the efficacy and safety of EG-1962 relative to standard care (oral nimodipine). In the study, the EG-1962 treatment group received a single 600 mg intraventricular injection of EG-1962 while oral placebo capsules or tablets were administered daily until day 21. The control group received a single intraventricular injection of normal saline while orally taking nimodipine capsules or tablets daily until day 21. The primary endpoint of the study was the proportion of patients with a Glasgow Prognostic Scale (GOSE) score of 6-8 on Day 90.
The interim analysis data was derived from the statistics of the first batch of 210 patients on the 90th day after treatment. The data showed that even though the study recruited full staff, the probability of a statistically significant difference in EG-1962 relative to standard care was low and it was unlikely to reach the primary end point of the study.
At the same time as the research data was released, Edge also announced that it will begin to reduce its business scope, including layoffs, in order to maintain its cash resources. The company said that as of December 31, 2017, the company's cash was only $88.1 million.
An independent data monitoring committee (DMC) has recommended termination of the study based on the analysis. Edge has complied with the DMC's recommendations and has initiated procedures to notify regulators and clinical research participants of this news. The company said it will analyze the double-blind data that has been obtained to better understand the reasons for this result.
Brian A. Leuthner, President and CEO of Edge, said that we were very disappointed that EG-1962 failed to show evidence of improved prognosis in the NEWTON-2 study. Previously, in the randomized open-label I/II study NEWTON, EG-1962 did show positive results in a similar patient population. We are very grateful to the patients involved in the study and their families and the team of researchers for their support.
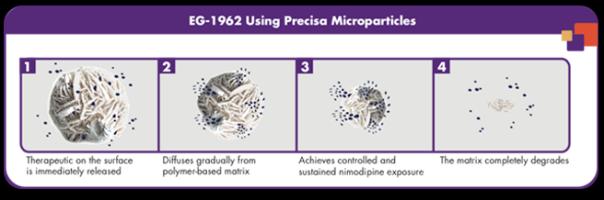
EG-1962 is a new dosage form of nimodipine. Nimodipine is mainly used to treat blood pressure related diseases such as soothing subarachnoid hemorrhage, cerebral insufficiency, and migraine. EG-1962 is a novel polymer microparticle in which nimodipine is suspended in sodium hyaluronate diluent and administered once by intraventricular drainage (EVD).
EG-1962 is based on Edge's proprietary Precisa Multimer Particle Technology Platform, which effectively increases the local delivery concentration of nimodipine, which is designed to improve the prognosis of patients after aSAH. Precisa is a proprietary, design, biodegradable, polymer-based development platform that uses the platform to create polymer drugs that can be delivered directly to the site of injury to avoid severe systemic effects associated with oral or intravenous administration. Sexual side effects. Such multimeric microparticle drugs require a single dose of treatment at the time of surgery to achieve high concentrations of sustained drug exposure at the site of injury.
aSAH is a type of brain hemorrhage, mainly due to the rupture of the aneurysm in the patient's brain, causing blood to enter the subarachnoid space, which has serious consequences. It is estimated that there are approximately 35,000 aSAHs per year in the United States, of which approximately 75% will die or have permanent brain damage.
Based on the enormous potential demonstrated in Phase I/II clinical trials, the FDA has previously granted EG-1962 a fast-track status, and the drug has also been granted orphan drug status in the United States and the European Union. The Fast Track is a program created by the FDA to promote drug development and speed up drug review, involving the treatment of serious or fatal diseases and showing the potential to address the unmet medical needs of such diseases. An important feature of the FDA Fast Track program is its emphasis on frequent communication between the FDA and drug developers throughout the drug development and review process to improve product development efficiency. Therefore, the fast track can potentially shorten the time for the final drug to be approved.
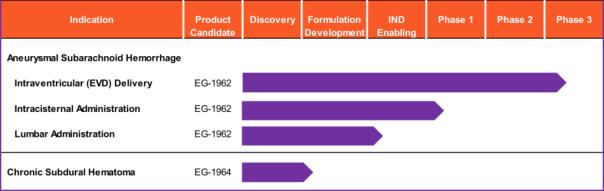
EG-1962 is the leading drug of Edge and the only drug to enter clinical research. This shocking reversal is no different from the disaster. In addition to EG-1962, Edge is currently developing a new drug, EG-1964, for the treatment of chronic subdural hematoma. The drug was also developed using the company's Precisa polymer microparticle technology platform, which contains anti-hemorrhagic drug aprotinin in degradable microparticles. The drug is currently in development.
The Edge company pipeline is as follows:
(Sina Pharmaceutical Compilation/newborn)
Original, image source: Edge plans job cuts as phase 3 brain-bleed fail routs stock
Aphrodisiacs refer to drugs used to promote male libido and other sexual functions, and are divided into aphrodisiac food, aphrodisiac Chinese and Western patent medicines, etc. With the acceleration of the current pace of life and the increase of work pressure, the number of patients with sexual dysfunction is increasing day by day, and aphrodisiac is one of the popular industries in medicine.
Our company specializes in providing raw materials for the treatment of ED, such as Tata, Jinyang alkali, Maca, Yohimbine, etc.

Saw Palmetto Powder,Maca Root Extract Powder,Polysaccharide Powder,Sexual Enhancement Materials
XI AN RHINE BIOLOGICAL TECHNOLOGY CO.,LTD , https://www.rhinebioteches.com