Strictly control the safety risks of imported medical devices
In order to prevent and control the risk of imported products, the State Food and Drug Administration has increased the scope and intensity of inspections on overseas production sites. In the field of medical devices, in 2017 alone, it has successively applied to 24 medical device manufacturers in 10 countries including the United States and Germany. 46 imported products (including 6 in-process products) carry out on-site production site inspections, involving orthopedic implants, vascular stents, staplers, vascular catheters, dressing products and in vitro diagnostic reagents.
On January 17, 2018, the official website of the State Food and Drug Administration announced the results of on-site inspections of nine medical device companies. This is the third batch of inspection results announced by the State Food and Drug Administration after the first overseas production site inspection results of five medical device companies were announced on November 28, 2017. Judging from the inspection results, the above-mentioned enterprises are mainly distributed in countries and regions with advanced industrial technology levels such as the United States and Europe. Their defects and problems involve product registration, plant and facilities, quality control, process flow, procurement, and document management. Among them, two companies were ordered to stop importing products due to violations.
Step by step
According to reports, in 2015, the State Food and Drug Administration organized personnel to conduct on-site inspections of two overseas medical device manufacturers. In 2016, a total of 33 varieties of 19 medical device manufacturers in 8 countries were inspected on the production site. In 2017, production inspections were carried out on 46 products (including 6 in-process products) of 24 companies in 10 countries including the United States, Germany, and the United Kingdom. In 2018, the number of inspection enterprises will reach more than 30, and the inspection intensity will increase year by year.
"From the perspective of the entire operation process, the first year was a pilot, so we chose Johnson & Johnson, which is a large-scale technology and management leader, mainly based on learning experience and ways of exploring work. In the second year, the scope of inspection was expanded. The inspection results were not announced. It was not until the end of 2017 that the results of overseas production site inspections were published on the website of the State Administration of Taxation, indicating that the overseas inspection of medical equipment in China has been on the right track.†The State Food and Drug Administration Food and Drug Inspection and Inspection Center (hereinafter referred to as “ Wang Aijun, director of the Medical Device Verification Division, said that “the results of overseas production site inspections of 24 companies inspected in 2017 will be published online. The penalties will be imposed on the illegal activities that the company does.â€
According to the "Regulations on the Overseas Inspection of Medical Devices" issued by the State Food and Drug Administration on December 9, 2016, before November 30 of each year, the State Food and Drug Administration will complete the annual review of the annual plan for overseas inspection of medical devices, including the annual Check the proposing, confirmation, and approval of the variety, and convene the ventilation meeting of the manufacturer of the inspected variety. The verification center selects qualified inspectors according to the product category characteristics, and combines the production area and inspection time of the products to prepare an annual inspection plan and submit it to the Medical Device Supervision Department of the State Food and Drug Administration. After approval by the head of the General Administration, it is reported to the Department of International Cooperation of the General Administration. .
“All enterprises that are included in the annual inspection plan must open a ventilation meeting to determine which country the product is in, the specific production status of the product, etc.†Wang Aijun said that the formulation of the inspection plan is not simple. In many cases, the foreign registered address and release address of the product are not in one place, and the main production links need to be determined before the inspection plan can be determined.
Self-pressurizing
According to reports, after the inspection plan is finalized, the inspector's determination also needs to consider a variety of factors.
“Overseas inspection, inspector qualifications, staffing, and inspection methods are different from those in the country.†Wang Aijun told reporters that generally every breed or enterprise should be inspected for 4 to 5 days, and each group usually sends 3 to 5 inspectors. It covers the professions of testing, registration, review, system inspection, etc., and sometimes includes the staff of the Medical Device Registration Management Department or the Medical Device Supervision Department of the State Food and Drug Administration.
Overseas inspections have a large workload, high professional requirements, and many unexpected situations. Therefore, it is a big challenge for inspectors.
Zhao Guangyu, the national equipment inspector who participated in the overseas production site inspection for the first time in 2015, said that the overseas inspection needs to declare the plan in advance, and also according to the requirements of foreign affairs work, and wait for the other party to issue an invitation letter. Due to the need to coordinate the reporting of overseas indicators, and the foreign affairs procedures take a long time, the random and non-informative overseas inspections have not yet been implemented, which also restricts the effectiveness and scale of the inspection to some extent.
Li Xintian, who led the team to Japan for on-site inspections, said that due to differences in language, cognition, culture, customs, etc., even if sufficient preparations and plans were made before the trip, there may be various emergencies at the inspection site. For example, the production address change, commissioned production, and quality management system related functions are decomposed into multiple related or unrelated companies.
"Inspectors are experiencing the ability to adapt abroad and handle communication skills at all times," Li Xintian said.
Decomposition task perfection procedure
In October 2017, the “Opinions on Deepening the Reform of the Examination and Approval System and Encouraging the Innovation of Pharmaceutical Medical Devices†issued by the State Council and the State Council (hereinafter referred to as the “Opinionsâ€) put forward general requirements for the supervision and examination of medical devices.
It is understood that the State Food and Drug Administration, based on actual conditions, has scientifically designed a system for the division of medical device supervision and inspection rights on the basis of in-depth investigations, and insisted on assigning supervision and inspection tasks according to the supervision power at all levels to guide local supervision and inspection, and strengthen the focus. On-site inspection of overseas production enterprises to ensure that inspection tasks are not lost.
In order to standardize the overseas inspection of pharmaceutical medical devices, the State Food and Drug Administration issued the "Regulations on the Administration of Overseas Inspection of Pharmaceutical Medical Devices (Draft for Comment)" in December 2017, the general rules for the overseas inspection of pharmaceutical medical devices, and the determination of inspection tasks. The process, inspection process, audit and treatment processes and principles, as well as related regulations and annexes, are clearly defined.
Wang Aijun said that to strengthen the on-site inspection of medical equipment overseas production, on the one hand, it can urge foreign production enterprises to abide by the relevant regulations of China's medical device production quality management regulations, and also exercise the international inspection capability and internationalization level of medical device inspectors in China.
However, according to the new medical device regulatory authority, in the face of more than 4,000 overseas enterprises and the annual flight inspection tasks of hundreds of domestic production and operation enterprises, relying on the existing part-time inspectors is far from sufficient.
It is reported that in the next step, the Medical Device Supervision Department of the State Food and Drug Administration will speed up the implementation of the "Opinions" requirements, issue guidance, promote the establishment of a full-time inspector at the national and provincial levels, and train inspectors with high-level international inspection capabilities. We will strive to achieve the goal of full coverage inspection of imported high-risk medical device products at an early date.
(Source: China Medical News)
Printing Glow In Dark Tape
1. Material and classification of glow in dark tape
We have PET material glow in dark tape and PVC material glow in dark tape. PET material is cheaper but not printable. PVC material support customized printing with low MOQ.
For both materials, we have different kinds according to the glowing time it can last after full charging (0.5hours charging at least). We have 2hours, 4hour, 6hours, 8hours and 10hours according to the glowing period it can last.
2. Colors for choosing
We have light green, pink, orange, red, blue, white for choosing.
3. Features
a. Good ahesion, we use solvent acrylic adhesive, the adhesive is strong and long lasting.
b. Waterproof. Both PET and PVC material are waterproof. Can used for both indoor and outdoor usage.
c. Long service life: 2~3 years even for outdoor usage.
d. Different sizes for choosing: 1.24m x 45.7m log roll, or other customized sizes such as 25mm/ 50mm x 5meters/ 10yards/ 10meters/ 18meters, etc.
e, Accept die cuting to small pieces such as dots, stars, arrows, etc,







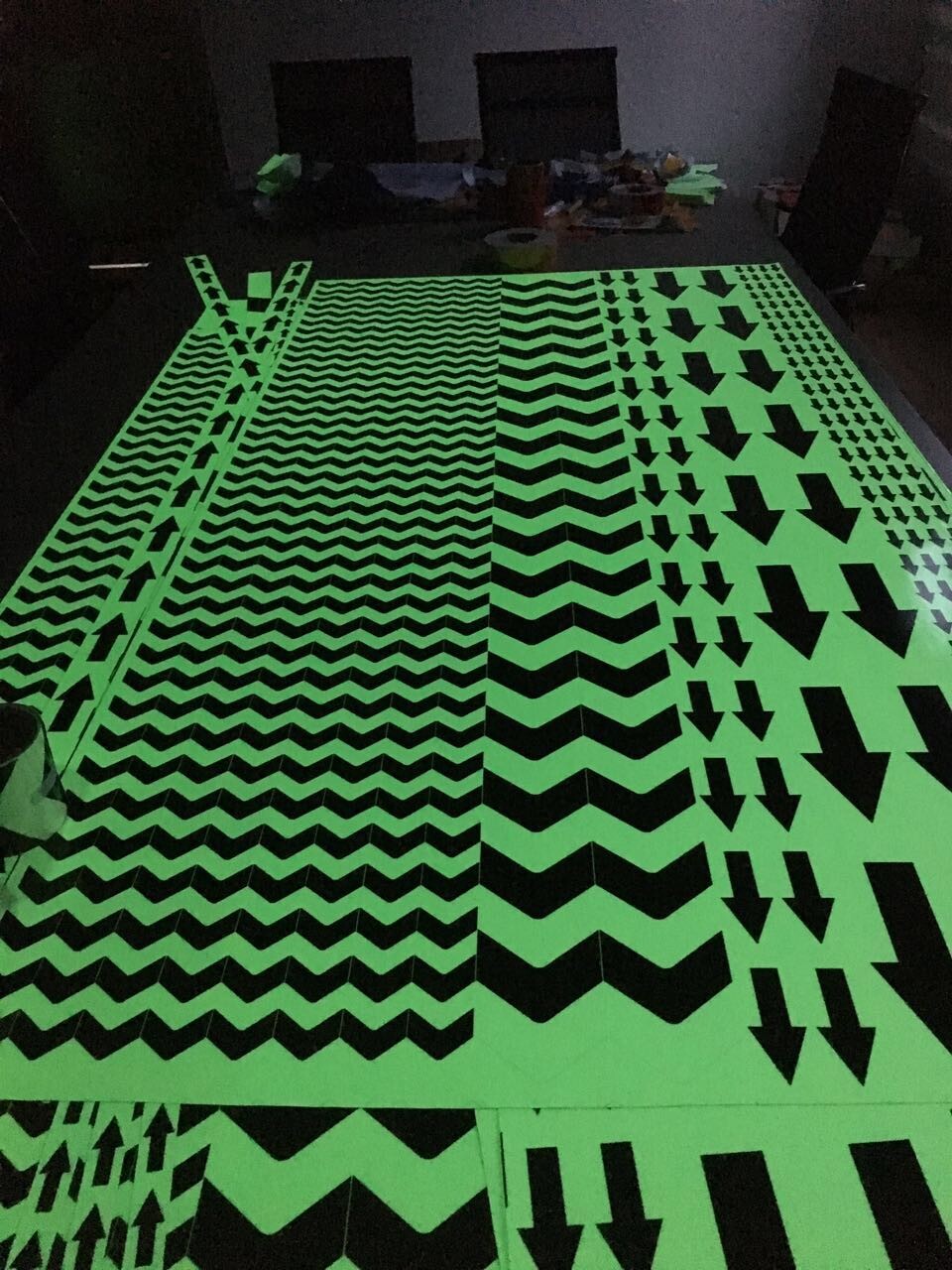
Printing Glowing Tape,Printing Glow In Dark Tape, Customized glow in dark tape, customized luminous tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.yuhuanptapes.com